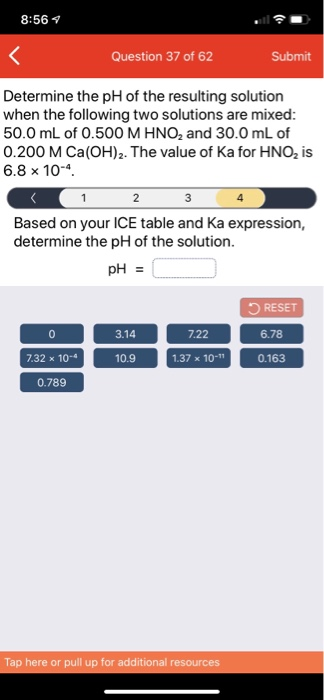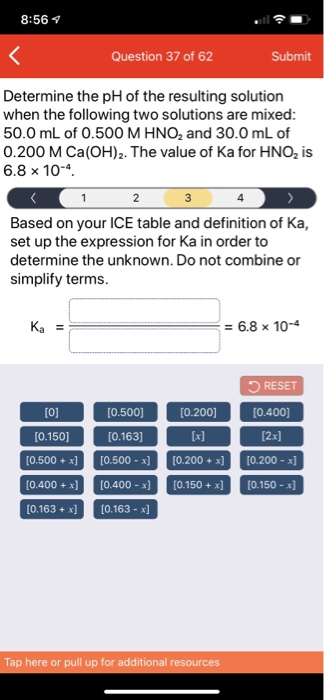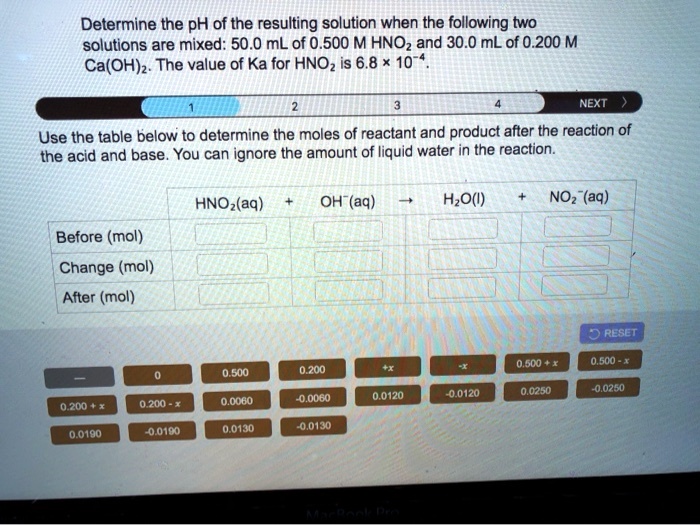
SOLVED: Determine the pH of the resulting solution when the following two solutions are mixed: 50.0 mL of 0.500 M HNOz and 30.0 mL of 0.200 M Ca(OH)z: The value of Ka

Equal volumes of two HCl solutions of pH=3 and pH=5 were mixed. What is the Ph of the resulting solution ?

Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.
The equal volume of two HCL solutions of pH=3 and pH=5 were mixed. What is the pH of the resulting solution? - Quora

Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.